BIOMET BRINGS PERSONALIZED APPROACH TO PARTIAL KNEE REPLACEMENTS
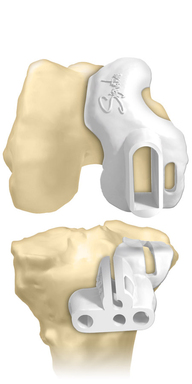
Biomet Orthopedics today announced the launch of the Signature™ Personalized Patient Care System for use with the Oxford® Partial Knee System. The Signature™ Personalized Patient Care system enables surgeons to preoperatively plan a knee replacement surgery and precisely place implants by using Signature™ custom positioning guides.
“With Signature™ and the Oxford® Partial Knee, surgeons now have state-of-the-art technology for patients undergoing partial knee replacement surgery,” said Dr. Michael Berend from the Center for Hip & Knee Surgery at St. Francis Health Hospital – Mooresville in Indianapolis.
“With Signature™ and the Oxford® Partial Knee, surgeons now have state-of-the-art technology for patients undergoing partial knee replacement surgery,” said Dr. Michael Berend from the Center for Hip & Knee Surgery at St. Francis Health Hospital – Mooresville in Indianapolis.
Biomet’s Oxford® Partial Knee System is designed to help surgeons to preserve and restore normal knee function and movement by replacing only the medial, diseased compartment of the knee. During this process, surgeons use the Signature™ system to create custom femoral and tibial surgical positioning guides that help achieve optimal joint implant positioning. The Oxford® Partial Knee procedure removes approximately 75% less bone and cartilage when compared to a total knee replacement and allows patients to recover more rapidly with less postoperative pain.4-6 The Oxford® Partial Knee is intended for use in individuals with osteoarthritis or avascular necrosis limited to the medial compartment of the knee and is intended to be implanted with bone cement. The Oxford® Knee is not indicated for use in the lateral compartment. Potential risks include, but are not limited to, loosening, dislocation, fracture, wear, and infection, any of which can require additional surgery. For additional information about the Signature™ system and the Oxford® Knee, including risks and warnings, talk to your surgeon and see the full patient risk information on http://www.biomet.com.
Biomet is one of the first orthopedic implant companies to provide surgeons advanced tools to create custom femoral and tibial positioning guides for use in both total and partial knee replacement. Introduced in 2008, the Signature™ System has been used in more than 41,000 knee surgeries to date. By combining this technology with the Oxford® Partial Knee, the most clinically successful partial knee in the world with survivorship of 92.3% at 20 years,7 Biomet provides surgeons and their patients the advanced technology necessary to deliver a higher level of personalized care in partial knee replacement surgery.
For more information on the Signature™ Personalized Care System and Oxford® Partial Knee System, please visit: http://www.biomet.com.
About Biomet
Biomet, Inc. and its subsidiaries design, manufacture and market products used primarily by musculoskeletal medical specialists in both surgical and non-surgical therapy. Biomet’s product portfolio encompasses large joint reconstructive products, including orthopedic joint replacement devices, and bone cements and accessories; sports medicine, extremities and trauma products, including internal and external orthopedic fixation devices; spine and bone healing products, including spine hardware, spinal stimulation devices, and orthobiologics, as well as electrical bone growth stimulators and softgoods and bracing; dental reconstructive products; and other products, including microfixation products and autologous therapies. Headquartered in Warsaw, Indiana, Biomet and its subsidiaries currently distribute products in approximately 90 countries.
* A partnership with Materialise N.V.
1 “Knee Replacements Up Dramatically Among Adults 45 to 64 Years Old.” Agency for Healthcare Research and Quality. November 3, 2011. Accessed from http://www.ahrq.gov/news/nn/nn110311.htm
2 HCUPnet database, available at http://hcupnet.ahrq.gov
3 Kurtz, Steven M., Ph.D., et al. Future Young Patient Demand for Primary and Revision Joint Replacement. Clinical Orthopedics and Related Research (2009) 467:2606-2612.
4 Data on file at Biomet. Note: bench test results do not necessarily indicate clinical performance.
5 Deshmukh, RV, Scott, RD. "Unicompartmental knee Arthroplasty: long-term results." Clinical Orthopedics and Related Research. 2001; 392:272-278.
6 Murray, DW. "Mobile Bearing Unicompartmental Knee Replacement." Orthopedics. 2005:28:985-987.
7 Price AJ, Svard U. A second decade lifetable survival analysis of the Oxford unicompartmental knee arthroplasty. Clin Orthop Res. 2011; 469:174-179.

Комментариев нет:
Отправить комментарий